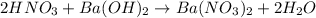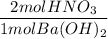Chemistry, 16.03.2020 06:22 kameronstebbins
If 10.0 mL of a .600 M of HNO3 reacts with 31.0 mL of .700M Ba(OH)2 solution, what is the molarity of Ba(OH)2 after the reaction is complete

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 23:30
What are the similarities between compounds and mixtures?
Answers: 3

Chemistry, 23.06.2019 01:30
Some molecular compounds, such as hcl, ionize completely in solution. for molecular compounds such as h2co3, most molecules do not ionize in solution. which describes the properties of these two solutes? a. hcl and h2co3 have the same effect on the properties of the solution. b. hcl raises the freezing point of water more than h2co3 does. c. hcl raises the boiling point of water more than h2co3 does.
Answers: 2

Chemistry, 23.06.2019 03:30
In chemistry, the type of an atom (what element it is) is determined by: a) the number of protons it contains in its nucleus.b) the number of neutrons it contains in its nucleus.c) the number of protons it has in a cloud around the nucleus.d) the number of neutrons it has in a cloud around the nucleus.e) the number of electrons it exchanges with its neighbors.
Answers: 1

Chemistry, 23.06.2019 09:00
How many moles of potassium hydroxide are needed to completely react with 2.94 moles of aluminum sulfate according to the following equation:
Answers: 2
You know the right answer?
If 10.0 mL of a .600 M of HNO3 reacts with 31.0 mL of .700M Ba(OH)2 solution, what is the molarity o...
Questions

Mathematics, 31.07.2020 21:01



Mathematics, 31.07.2020 21:01

Mathematics, 31.07.2020 21:01


Mathematics, 31.07.2020 21:01












Mathematics, 31.07.2020 21:01

Mathematics, 31.07.2020 21:01