Chemistry, 13.03.2020 18:34 RoxanneDuartee
The rate constants of some reactions double with every 10-degree rise in temperature. Assume that a reaction takes place at 295 K and 305 K. What must the activation energy be for the rate constant to double as described?

Answers: 1
Another question on Chemistry


Chemistry, 21.06.2019 20:30
18. use the activity series to predict whether the following synthesis reaction will occur. write the chemical equations for the reaction if it's predicted to occur. (s) + o2(g) -> *note: it is possible.*
Answers: 1

Chemistry, 22.06.2019 11:10
Which of the following shapes would represent a molecule with two bonded atoms and 3 lone pairs on only one of them , trigonal planar , bent , trigonal pyramidal , linear
Answers: 1

Chemistry, 22.06.2019 13:30
Which of the following has wavelength longer than the wavelength of viable light? a) x rays b) gamma rays c) radios waves d) ultraviolet waves
Answers: 1
You know the right answer?
The rate constants of some reactions double with every 10-degree rise in temperature. Assume that a...
Questions

Health, 26.08.2019 21:10

History, 26.08.2019 21:10


Mathematics, 26.08.2019 21:10
















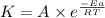
![\log (\frac{K_2}{K_1})=\frac{Ea}{2.303\times R}[\frac{1}{T_1}-\frac{1}{T_2}]](/tpl/images/0546/6834/6d953.png)
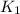 = rate constant at 295 K
= rate constant at 295 K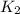 = rate constant at 305 K =
= rate constant at 305 K = 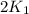
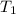 = initial temperature = 295 K
= initial temperature = 295 K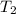 = final temperature = 305 K
= final temperature = 305 K![\log (\frac{2K_1}{K_1})=\frac{Ea}{2.303\times 8.314J/mole.K}[\frac{1}{295K}-\frac{1}{305K}]](/tpl/images/0546/6834/d1fff.png)