
Chemistry, 13.03.2020 17:28 ramentome7542
You have an aqueous solution of chromium(III) nitrate that you titrate with an aqueous solution of sodium hydroxide. After a certain amount of titrant has been added, you observe a precipitate forming. You add more sodium hydroxide solution and the precipitate dissolves, leaving a solution again. What has happened?1) The precipitate was chromium hydroxide, which then reacted with more hydroxide to produce a soluble complex ion, Cr(OH)4?.2) The precipitate was chromium hydroxide, which dissolved once more solution was added, forming Cr3+(aq).3) The precipitate was sodium nitrate, which reacted with more nitrate to produce the soluble complex ion Na(NO3)2?.4) The precipitate was sodium hydroxide, which re-dissolved in the larger volume.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 06:30
Three cards with holes are arranged in a straight line. a light is shined through the first card’s hole and travels through all three cards. what does this tell you about light rays? a) that light is reflected b) that light is refractive c) that light travels in a straight line d) that light does not travel in a straight line
Answers: 1


Chemistry, 22.06.2019 09:10
How have the greenhouse gasses increased from the year 2000 to 2018
Answers: 2

Chemistry, 22.06.2019 14:50
How are evaporation and sublimation similar? a both involve the formation of a gas. b both release energy to the surroundings. c both take place throughout a solid. d both take place at the surface of a liquid.
Answers: 1
You know the right answer?
You have an aqueous solution of chromium(III) nitrate that you titrate with an aqueous solution of s...
Questions




Mathematics, 06.01.2020 15:31

History, 06.01.2020 16:31

Mathematics, 06.01.2020 16:31

Mathematics, 06.01.2020 16:31



Mathematics, 06.01.2020 16:31






English, 06.01.2020 16:31


Biology, 06.01.2020 16:31

Arts, 06.01.2020 16:31

Mathematics, 06.01.2020 16:31

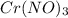 +3 NaOH →
+3 NaOH → 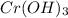 (s)+
(s)+ 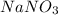
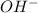 (aq) →
(aq) → 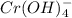 (aq)
(aq)

