
Chemistry, 12.03.2020 06:06 amohammad6
A basic solution contains the iodide and phosphate ions that are to be separated via selective precipitation. the i– concentration, which is 9.00×10-5 m, is 10,000 times less than that of the po43– ion at 0.900 m . a solution containing the silver(i) ion is slowly added. answer the questions below. ksp of agi is 8.30×10-17 and of ag3po4, 8.90×10-17.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 14:00
The two naturally occurring isotopes of chlorine are 35cl (34.969 amu, 75.77%) and 37cl (36.966 amu, 24.23%). the two naturally occurring isotopes of bromine are 79br (78.918 rm amu, 50.69%) and 81br (80.916 amu, 49.31%). chlorine and bromine combine to form bromine monochloride, brcl. 1. how many peaks will be present in a mass spectrum for brcl? the four combinations of molecule possible given these four isotopes are: 81br37cl, 81br35cl, 79br37cl, and 79br35cl. 2. what are the masses of the four different brcl molecules? express the masses using six significant figures, in decreasing numeric order (highest to lowest), separated by commas.
Answers: 3

Chemistry, 22.06.2019 21:00
As we move from left to right across the periodic table, what is the general trend? a) atomic radii increase. b) electronegavitiy decreases. c) nuclear shielding increases. d) metallic character decreases.
Answers: 1

Chemistry, 23.06.2019 03:00
Is it safe to take 450mg of diphenhydramine hydrochloride?
Answers: 1

Chemistry, 23.06.2019 03:30
The semi-conductors on the periodic table are classified as
Answers: 1
You know the right answer?
A basic solution contains the iodide and phosphate ions that are to be separated via selective preci...
Questions




English, 09.10.2019 18:00

Mathematics, 09.10.2019 18:00

Mathematics, 09.10.2019 18:00



Biology, 09.10.2019 18:00

History, 09.10.2019 18:00

Mathematics, 09.10.2019 18:00


Social Studies, 09.10.2019 18:00


Physics, 09.10.2019 18:00



History, 09.10.2019 18:00

Physics, 09.10.2019 18:00

History, 09.10.2019 18:00

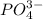 ion at 0.900 m . a solution containing the silver(i) ion is slowly added. answer the questions below. ksp of agi is 8.30×10-17 and of
ion at 0.900 m . a solution containing the silver(i) ion is slowly added. answer the questions below. ksp of agi is 8.30×10-17 and of 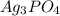 ,
, 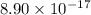 .
.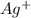 concentration required to cause precipitation of AgI.
concentration required to cause precipitation of AgI.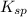 is equal to the ionic product.
is equal to the ionic product.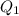 is as follows.
is as follows.![K_{sp} = [Ag^{+}][I^{-}]](/tpl/images/0544/6507/7f793.png)
![[I^{-}] = 7.7 \times 10^{-5}](/tpl/images/0544/6507/26cb8.png)
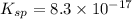
![[Ag^{+}] = \frac{k_{sp}}{I^{-}}](/tpl/images/0544/6507/fac7f.png)
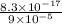
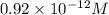
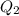 the expression for
the expression for ![K_{sp} = [Ag^{+}]^{3}[PO^{3-}_{4}]](/tpl/images/0544/6507/9aea6.png)
![[Ag^{+}] = (\frac{K_{sp}}{[PO^{3-}_{4}]})^{\frac{1}{3}}](/tpl/images/0544/6507/33b2e.png)
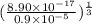
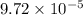 M
M 

