Chemistry, 12.03.2020 00:41 ayoismeisalex
A solution of hydrochloric acid of unknown concentration was titrated with 0.10 M NaOH. If a 100.-mL sample of the HCl solution required exactly 1.0 mL of the NaOH solution to reach the equivalence point, what was the initial pH of the HCl solution

Answers: 1
Another question on Chemistry


Chemistry, 22.06.2019 11:00
Imagine that twenty i.u.’s of enzyme z were catalyzing the above reaction for one minute, under vmaxconditions, in a 3.00 ml assay volume. the assay is buffered with 20 mm phosphate buffer, ph 7.60. what will the ph be at the end of that one minute?
Answers: 2

Chemistry, 22.06.2019 12:10
Consider the reaction: n2(g) + o2(g) ⇄ 2no(g) kc = 0.10 at 2000oc starting with initial concentrations of 0.040 mol/l of n2 and 0.040 mol/l of o2, calculate the equilibrium concentration of no in mol/l how would this be done?
Answers: 3

Chemistry, 22.06.2019 21:50
What is a main difference between a mixture and a pure substance? a mixture is only a liquid, but a pure substance can be in any state.a mixture looks the same throughout, but a pure substance does not.1 a mixture can vary in composition, but a pure substance has a set composlo a mixture can be made up of a single compound, but a pure substance car
Answers: 2
You know the right answer?
A solution of hydrochloric acid of unknown concentration was titrated with 0.10 M NaOH. If a 100.-mL...
Questions

Mathematics, 09.01.2021 04:20

History, 09.01.2021 04:20



Mathematics, 09.01.2021 04:20

Mathematics, 09.01.2021 04:20

Spanish, 09.01.2021 04:20


History, 09.01.2021 04:20

Mathematics, 09.01.2021 04:20




English, 09.01.2021 04:20

Mathematics, 09.01.2021 04:20

Mathematics, 09.01.2021 04:20

History, 09.01.2021 04:20



Social Studies, 09.01.2021 04:20

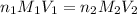
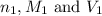 are the n-factor, molarity and volume of acid which is HCl
are the n-factor, molarity and volume of acid which is HCl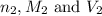 are the n-factor, molarity and volume of base which is NaOH.
are the n-factor, molarity and volume of base which is NaOH.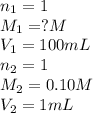
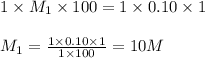
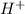 ions and 1 mole of
ions and 1 mole of 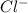 ions
ions![pH=-\log[H^+]](/tpl/images/0543/9407/cf945.png)
![[H^+]=0.001M](/tpl/images/0543/9407/58906.png)