
Chemistry, 11.03.2020 06:06 QuestionsAnsweredNow
A mixture of CO2 and Kr weighs 41.0 g and exerts a pressure of 0.729 atm in its container. Since Kr is expensive, you wish to recover it from the mixture. After the CO2 is completely removed by absorption with NaOH(s), the pressure in the container is 0.193 atm. (a) How many grams of CO2 were originally present? (b) How many grams of Kr can you recover?

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 11:00
Imagine that twenty i.u.’s of enzyme z were catalyzing the above reaction for one minute, under vmaxconditions, in a 3.00 ml assay volume. the assay is buffered with 20 mm phosphate buffer, ph 7.60. what will the ph be at the end of that one minute?
Answers: 2

Chemistry, 22.06.2019 11:50
Calculate the molarity of each of the following solutions. part a) 0.12 mol of lino3 in 5.5 l of solution part b) 60.7 g c2h6o in 2.48 l of solution part c) 14.2 mg ki in 100 ml of solution
Answers: 2

Chemistry, 22.06.2019 19:00
What information does a complete ionic equation give that the balanced equation doesn’t show?
Answers: 1

Chemistry, 22.06.2019 19:50
When the mercury level in a barometer decreases that atmospheric pressure has
Answers: 3
You know the right answer?
A mixture of CO2 and Kr weighs 41.0 g and exerts a pressure of 0.729 atm in its container. Since Kr...
Questions



English, 09.08.2021 20:50

Mathematics, 09.08.2021 20:50



Computers and Technology, 09.08.2021 20:50

English, 09.08.2021 20:50

Social Studies, 09.08.2021 20:50



Business, 09.08.2021 20:50

English, 09.08.2021 20:50



Computers and Technology, 09.08.2021 20:50




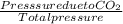
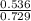
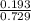
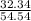 * 100 %
* 100 % 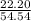 * 100 %
* 100 % 

