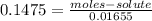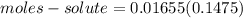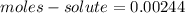Chemistry, 11.03.2020 02:26 graceception
Calculate the number of moles of solute in 16.55 mL of 0.1475 M M2Cr3O7

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 20:30
If a bottle of olive oil contains 1.4 kg of olive oil, what is the volume, in milliliters ( ml ), of the olive oil?
Answers: 1

Chemistry, 22.06.2019 07:00
The boiling point of propanoic acid is higher than that of 1-butanol because: propanoic acid has a higher molecular weight than 1-butanol. propanoic acid is more soluble in water than 1-butanol. propanoic acid is a better hydrogen bond donor than 1-butanol. propanoic acid forms hydrogen bonded dimers and 1-butanol does not. 1-butanol forms hydrogen bonded dimers and propanoic acid does not.
Answers: 2

Chemistry, 22.06.2019 20:20
The characteristics of two different types of reactions are shown below: reaction a: electrons are gained by the atoms of an element. reaction b: protons are lost by the atom of an element. which statement is true about the atoms of the elements that participate in the two reactions? their identity changes in both reaction a and reaction b. their identity changes in reaction a but not in reaction b. their identity changes in reaction b but not in reaction a. their identity remains the same in both reaction a and reaction b.
Answers: 1

Chemistry, 22.06.2019 23:00
Condensation happens when water vapor cools. true or false? ?
Answers: 2
You know the right answer?
Calculate the number of moles of solute in 16.55 mL of 0.1475 M M2Cr3O7...
Questions

Chemistry, 03.11.2020 05:20




English, 03.11.2020 05:20

French, 03.11.2020 05:20


Mathematics, 03.11.2020 05:20







Mathematics, 03.11.2020 05:20

English, 03.11.2020 05:20


Chemistry, 03.11.2020 05:20

Mathematics, 03.11.2020 05:20

 ,
,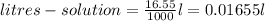 .
.