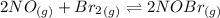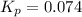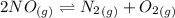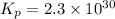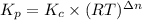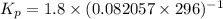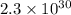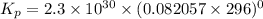Chemistry, 10.03.2020 09:56 mattmaddox86
Calculate the equilibrium constant Kp for this reaction, given the following information (at 296 K ): 2NO(g)+Br2(g)⇌2NOBr(g)Kc=1.8 2NO(g)⇌N2(g)+O2(g)Kc=2.3×1030

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 22:00
Describe the particles of all three phases of matter in the root beer float. (how are the particles arranged and moving? )
Answers: 3

Chemistry, 22.06.2019 01:30
Phosphorous acid, h3po3(aq) , is a diprotic oxyacid that is an important compound in industry and agriculture. the values of phosphorous acid are 1.30 6.70 calculate the ph for each of the given points in the titration of 50.0 ml of 1.5 m h3po3(aq) with 1.5 m koh(aq) .
Answers: 3


Chemistry, 22.06.2019 20:00
Many free radicals combine to form molecules that do not contain any unpaired electrons. the driving force for the radical–radical combination reaction is the formation of a new electron‑pair bond. consider the chemical equation. n(g)+no(g)⟶nno(g) n(g)+no(g)⟶nno(g) write lewis formulas for the reactant and product species in the chemical equation. include nonbonding electrons. n(g)n(g) select draw rings more erase select draw rings more erase select draw rings more erase n no(g)
Answers: 1
You know the right answer?
Calculate the equilibrium constant Kp for this reaction, given the following information (at 296 K )...
Questions

English, 10.09.2019 05:10

Mathematics, 10.09.2019 05:10

Mathematics, 10.09.2019 05:10


Biology, 10.09.2019 05:10

Health, 10.09.2019 05:10

Biology, 10.09.2019 05:10


Mathematics, 10.09.2019 05:10

English, 10.09.2019 05:10

Mathematics, 10.09.2019 05:10

Mathematics, 10.09.2019 05:10

History, 10.09.2019 05:10

Health, 10.09.2019 05:10



Mathematics, 10.09.2019 05:10