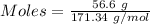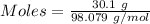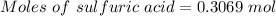For the following reaction, 56.6 grams of barium hydroxide are allowed to react with 30.1 grams of sulfuric acid. barium hydroxide (aq) + sulfuric acid (aq) barium sulfate (s) + water (l) What is the maximum amount of barium sulfate that can be formed? grams What is the FORMULA for the limiting reagent? What amount of the excess reagent remains after the reaction is complete? grams

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 23:30
Calculate the expected ph values of the buffer systems from the experiments (a,b,c,d), using the henderson- hasselbalch equation, ph-pka+log[a-]/[ha]. use for pka values carbonic acid= 6.37, and acetic acid= 4.75.
Answers: 2

Chemistry, 22.06.2019 08:00
An electron moved from shell n = 2 to shell n = 1. what most likely happened during the transition? a fraction of a photon was added. a photon of energy was absorbed. a fraction of a photon was removed. a photon of energy was released.
Answers: 1

Chemistry, 22.06.2019 12:10
|using the periodic tablewarm-upuse the periodic table in the tools bar to answer the following questions.what elemental classification does oxygen belongto? done
Answers: 3

Chemistry, 22.06.2019 14:00
650.j is the same amount of energy as? 2720cal1550cal650.cal2.72cal
Answers: 2
You know the right answer?
For the following reaction, 56.6 grams of barium hydroxide are allowed to react with 30.1 grams of s...
Questions


History, 18.10.2019 16:50


Health, 18.10.2019 16:50

Biology, 18.10.2019 16:50



English, 18.10.2019 16:50




Mathematics, 18.10.2019 16:50

Physics, 18.10.2019 16:50

Social Studies, 18.10.2019 16:50






 .
.