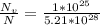Chemistry, 10.03.2020 07:42 gudon986732
For some hypothetical metal, the equilibrium number of vacancies at 600°C is 1 × 1025 m-3. If the density and atomic weight of this metal are 7.40 g/cm3 and 85.5 g/mol, respectively, calculate the fraction of vacancies for this metal at 600°C.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 00:00
1) this is the structure in the cell nucleus that houses a cell's genetic information
Answers: 3

Chemistry, 22.06.2019 05:30
Transportation is the largest single source of air pollution in the united states. air pollution can harm the environment and human health. which technology could offer a solution to this problem? mufflers that reduce noise motors that run on electricity tires that improve gas mileage
Answers: 3

Chemistry, 22.06.2019 15:00
Phosphorous acid, h3po3(aq) , is a diprotic oxyacid that is an important compound in industry and agriculture. p k a1 p k a2 1.30 6.70 calculate the ph for each of the points in the titration of 50.0 ml of 1.5 m h3po3(aq) 1.5 m h 3 po 3 ( aq ) with 1.5 m koh(aq). 1.5 m koh ( aq ) .
Answers: 1

Chemistry, 22.06.2019 21:20
40dm3 of gas at 760 torr are heated from 5°c to 50°c what is the new volume
Answers: 3
You know the right answer?
For some hypothetical metal, the equilibrium number of vacancies at 600°C is 1 × 1025 m-3. If the de...
Questions


Chemistry, 30.04.2021 14:00

Biology, 30.04.2021 14:00

World Languages, 30.04.2021 14:00



Mathematics, 30.04.2021 14:00


Mathematics, 30.04.2021 14:00

Mathematics, 30.04.2021 14:00



English, 30.04.2021 14:00

Physics, 30.04.2021 14:00

History, 30.04.2021 14:00

Mathematics, 30.04.2021 14:00

English, 30.04.2021 14:00

Mathematics, 30.04.2021 14:00


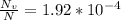
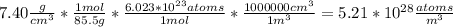
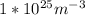 N is the number of atoms per volume calculated above.
N is the number of atoms per volume calculated above.