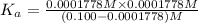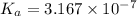Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 02:00
How many moles of magnesium is 3.01 x10^22 atoms of magnesium?
Answers: 1

Chemistry, 22.06.2019 03:00
Which of the dna typing techniques do you think you would choose if you had to analyze a dna sample? why?
Answers: 1

Chemistry, 22.06.2019 04:30
There is a single path for electrons. the current decreases when additional resistors are added. the current will be the same in each resistor. these statements best describe a(n) circuit.
Answers: 3

Chemistry, 22.06.2019 21:00
Kp is the equilibrium constant for dissociation of the propionic acid dimer. what is the sign of the slope for a plot of the natural logarithm of kp vs. inverse temperature for this reaction?
Answers: 1
You know the right answer?
A 0.100 mole quantity of a monoprotic acid HA is added to 1.00 L of pure water. When equilibrium is...
Questions







Health, 21.01.2020 22:31

History, 21.01.2020 22:31




Biology, 21.01.2020 22:31








 is the value of
is the value of 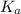 for the acid HA.
for the acid HA.![pH=-log[H^+]](/tpl/images/0540/0009/15713.png)
![3.75=-\log[H^+]](/tpl/images/0540/0009/128bb.png)
![[H^+]=10^{-3.75}=0.0001778 M](/tpl/images/0540/0009/6c87c.png)
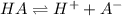
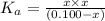
![[H^+]=0.0001778 M](/tpl/images/0540/0009/4f0b0.png)