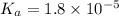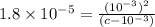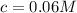Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 08:30
How does the principle of electromagnetism explain the interaction between earth’s magnetic field and the solar wind?
Answers: 1

Chemistry, 22.06.2019 09:20
Sugar is dissolved in water. which is the solute? sugar neither both water
Answers: 1

Chemistry, 22.06.2019 10:30
Apiece of metal with a length of 1.42 cm was measured using four different instruments. which of the following measurements is the most accurate?
Answers: 3

You know the right answer?
A typical sample of vinegar has a pH of 3.0. Assuming that vinegar is only an aqueous solution of ac...
Questions

Mathematics, 13.09.2019 21:30

English, 13.09.2019 21:30




History, 13.09.2019 21:30



Mathematics, 13.09.2019 21:30



Mathematics, 13.09.2019 21:30




History, 13.09.2019 22:10


Social Studies, 13.09.2019 22:10

Mathematics, 13.09.2019 22:10

History, 13.09.2019 22:10

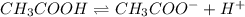
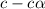
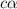
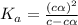
![pH=-log[H^+]](/tpl/images/0539/0805/15713.png)
![3.0=-log[H^+]](/tpl/images/0539/0805/d1dae.png)
![[H^+]=c\times \alpha=10^{-3}](/tpl/images/0539/0805/9452b.png)