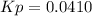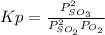Chemistry, 09.03.2020 23:46 TerronRice
A mixture of gaseous sulfur dioxide and oxygen are added to a reaction vessel and heated to 1000 K where they react to form SO3 (g). If the vessel contained 0.669 atm SO2 (g), 0.395 atm O2 (g) and 0.0851 atom SO3 (g) after the system has reached equilibrium, what is the equilibrium constant, Kp, for the reaction

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 01:30
Reaction rate depends on how many molecules are coming into contact with each other with enough energy to react. increasing the temperature of the reactants will increase -
Answers: 3

Chemistry, 22.06.2019 09:00
Ineed to find the answer of this question because i dont understand it
Answers: 1

Chemistry, 22.06.2019 23:30
Imagine a small synthetic vesicle made from pure phospholipids enclosing an interior lumen containing 1 mm glucose and 1 mm sodium chloride. if the vesicle is placed in pure water, which of the following happens faster? a. na+ diffuses out. b. cl– diffuses out. c. h2o diffuses in. d. glucose diffuses out. e. sodium chloride diffuses out.
Answers: 3

Chemistry, 23.06.2019 14:30
2.38g of black copper (ii) oxide is completely reduced by hydrogen to give copper and water. what are the masses of copper and water formed? ?
Answers: 1
You know the right answer?
A mixture of gaseous sulfur dioxide and oxygen are added to a reaction vessel and heated to 1000 K w...
Questions


Mathematics, 14.07.2020 02:01


Mathematics, 14.07.2020 02:01

Chemistry, 14.07.2020 02:01

Mathematics, 14.07.2020 02:01

English, 14.07.2020 02:01


Chemistry, 14.07.2020 02:01

Mathematics, 14.07.2020 02:01

Mathematics, 14.07.2020 02:01


English, 14.07.2020 02:01



Mathematics, 14.07.2020 02:01

Mathematics, 14.07.2020 02:01



Mathematics, 14.07.2020 02:01