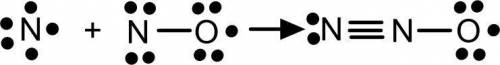Chemistry, 07.03.2020 05:58 catsareokiguess
Many free radicals combine to form molecules that do not contain any unpaired electrons. The driving force for the radical–radical combination reaction is the formation of a new electron‑pair bond. Consider the chemical equation. N ( g ) + NO ( g ) ⟶ NNO ( g ) Write Lewis formulas for the reactant and product species in the chemical equation. Include nonbonding electrons.

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 19:00
Does the temperature affect the solubility of sugar and salt in water? if it does tell me like different temperatures with different solubilities so i can sketch down a graph
Answers: 2

Chemistry, 21.06.2019 22:30
Which statement best describes the oxidation numbers of the atoms found in magnesium chloride? a. magnesium has a 2- oxidation number and chlorine has a 1+ oxidation number. b. magnesium has a 2- oxidation number and chlorine has a 2+ oxidation number. c. magnesium has a 2+ oxidation number and chlorine has a 1- oxidation number. d. magnesium has a 1+ oxidation number and chlorine has a 1- oxidation number.
Answers: 2

Chemistry, 22.06.2019 21:00
One similarity and one difference between an element and a mixture of elements
Answers: 1

Chemistry, 22.06.2019 21:00
Which property of water causes water drops to bead on a freshly waxed car?
Answers: 2
You know the right answer?
Many free radicals combine to form molecules that do not contain any unpaired electrons. The driving...
Questions

Mathematics, 24.04.2020 18:56



Mathematics, 24.04.2020 18:56


Mathematics, 24.04.2020 18:56


Social Studies, 24.04.2020 18:56





History, 24.04.2020 18:56

English, 24.04.2020 18:56






Advanced Placement (AP), 24.04.2020 18:56