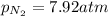Chemistry, 07.03.2020 04:48 Mattisback2285
A chemist fills a reaction vessel with 7.92 atm nitrogen (N2) gas, 2.02 atm hydrogen (H2) gas, and 2.11 atm ammonia (NH3) gas at a temperature of 25.0°C
Under these conditions, calculate the reaction free energy ΔG for the following chemical reaction
N2(8) +3H2 2NH3 (g)
Use the thermodynamic information in the ALEKS Data tab. Round your answer to the nearest kilojoule.

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 14:00
Write skeleton equations for the following reactions c. aluminum(s)+copper(i) chloride(aq) > aluminum chloride(aq)+copper(s)
Answers: 1

Chemistry, 22.06.2019 08:30
The mass of a neutron is equal to the mass of a proton plus the mass of an electron. true or false false true
Answers: 1

Chemistry, 22.06.2019 10:10
How do you identify the anode on a power source such as a battery? how do you identify the cathode? how are terms anion and cation?
Answers: 1

You know the right answer?
A chemist fills a reaction vessel with 7.92 atm nitrogen (N2) gas, 2.02 atm hydrogen (H2) gas, and 2...
Questions


SAT, 02.02.2022 17:20




Mathematics, 02.02.2022 17:30



Business, 02.02.2022 17:30

Business, 02.02.2022 17:30



Mathematics, 02.02.2022 17:30




English, 02.02.2022 17:30



Mathematics, 02.02.2022 17:30


![\Delta G^o_{rxn}=[(2\times \Delta G^o_f_{(NH_3(g))})]-[(1\times \Delta G^o_f_{(N_2(g))})+(3\times \Delta G^o_f_{(H_2(g))})]](/tpl/images/0537/7676/6e73c.png)

![\Delta G^o_{rxn}=[(2\times (-16.45))]-[(1\times (0))+(3\times (0))]\\\\\Delta G^o_{rxn}=-32.9kJ/mol](/tpl/images/0537/7676/8bdb6.png)

 = free energy of the reaction
= free energy of the reaction = standard Gibbs free energy = -32.9 kJ/mol = -32900 J/mol (Conversion factor: 1 kJ = 1000 J)
= standard Gibbs free energy = -32.9 kJ/mol = -32900 J/mol (Conversion factor: 1 kJ = 1000 J)![25^oC=[273+25]K=298K](/tpl/images/0537/7676/0e82f.png)
 = Ratio of concentration of products and reactants at any time =
= Ratio of concentration of products and reactants at any time =