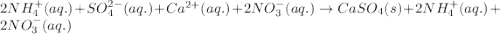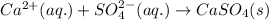Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 14:30
Select the word from the list that best fits the definition the nuclear family into which a person is born or adopted.
Answers: 2

Chemistry, 22.06.2019 23:00
Condensation happens when water vapor cools. true or false? ?
Answers: 2

Chemistry, 23.06.2019 02:50
What is the typical rotational frequency frot for a molecule like n2 at room temperature (25∘c)? assume that d for this molecule is 1å=10−10m. take the total mass of an n2 molecule to be mn2=4.65×10−26kg. you will need to account for rotations around two axes (not just one) to find the correct frequency. express frot numerically in hertz, to three significant figures.
Answers: 3

Chemistry, 23.06.2019 07:20
Which of the following are acids or bases? 1. sodium hydrogen 2. barium hydroxide solution 3. carbonate solution
Answers: 1
You know the right answer?
An aqueous solution of ammonium sulfate is allowed to react with an aqueous solution of calcium nitr...
Questions

Mathematics, 26.08.2021 02:50

History, 26.08.2021 02:50


Mathematics, 26.08.2021 02:50




English, 26.08.2021 02:50


Computers and Technology, 26.08.2021 02:50

Mathematics, 26.08.2021 02:50


Mathematics, 26.08.2021 02:50


Mathematics, 26.08.2021 02:50





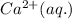 ions
ions