
Chemistry, 06.03.2020 23:26 lilquongohard
You carefully weigh out 16.00 g of CaCO3 powder and add it to 64.80 g of HCl solution. You notice bubbles as a reaction takes place. You then weigh the resulting solution and find that it has a mass of 74.24 g . The relevant equation is CaCO_3(s) + 2HCl(aq) rightarrow H_2O(l) + CO_2(g) + CaCl_2(aq) Assuming no other reactions take place, what mass of CO_2 was produced in this reaction?

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 02:00
In which of these cases are the two wave points considered to be in phase with each other?
Answers: 1

Chemistry, 22.06.2019 06:30
Use examples from the article to explain one positive and one negative effect that chemistry has had on society.
Answers: 2

Chemistry, 23.06.2019 07:50
Asolution is produced in which water is the solvent and there are four solutes. which of the solutes can dissolve better if the solution is heated?
Answers: 1

Chemistry, 23.06.2019 08:40
The half-life of a certain element is 100 days. how many half-lives will it be before only one eighth of this elementremains?
Answers: 1
You know the right answer?
You carefully weigh out 16.00 g of CaCO3 powder and add it to 64.80 g of HCl solution. You notice bu...
Questions

Mathematics, 01.07.2020 16:01








Social Studies, 01.07.2020 16:01

Mathematics, 01.07.2020 16:01


English, 01.07.2020 16:01








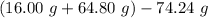
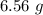
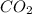 produced in this reaction was 6.56 grams
produced in this reaction was 6.56 grams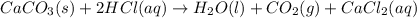
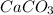 + mass of
+ mass of 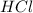 = 16.00 + 64.80 = 80.80 g
= 16.00 + 64.80 = 80.80 g


