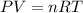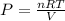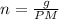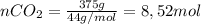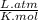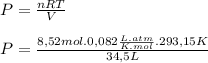Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 19:30
Agas has a volume of 0.7 l at 300 mmhg. what would be the new volume at 900 mmhg
Answers: 1

Chemistry, 22.06.2019 05:30
What is the mass of each element in a 324.8 sample of co2
Answers: 1

Chemistry, 22.06.2019 07:30
The volume of helium in a blimp is 6.28 x 10^9 millimeters. the density of helium in the blimp is .1786 kilogram/meter^3. find the mass of the helium in the blimp.
Answers: 1

Chemistry, 22.06.2019 12:00
An atom's configuration based on its number of electrons ends at 3p4. another atom has seven more electrons. starting at 3p, what is the remaining configuration? 3p63d34s2 3p43d54s2 3p64s23d3 3p44s23d
Answers: 3
You know the right answer?
If 375 grams of CO2 are placed in a vessel whose volume is 34.5 liters at a temperature of 20 degree...
Questions

Mathematics, 21.01.2021 02:50

Biology, 21.01.2021 02:50

Mathematics, 21.01.2021 02:50

Chemistry, 21.01.2021 02:50

Mathematics, 21.01.2021 02:50

Biology, 21.01.2021 02:50



Biology, 21.01.2021 02:50

Physics, 21.01.2021 02:50

Mathematics, 21.01.2021 02:50




Business, 21.01.2021 02:50

History, 21.01.2021 02:50

Health, 21.01.2021 02:50

Mathematics, 21.01.2021 02:50

Mathematics, 21.01.2021 02:50

Mathematics, 21.01.2021 02:50