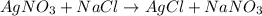Chemistry, 05.03.2020 00:22 tytybruce2
On a balance, you have beakers of AgNO3AgNO3 solution and NaClNaCl solution. When mixed, they will form AgCl(s)AgCl(s). What will happen to the mass of the new mixture?

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 10:00
Water's surface tension and heat storage capacity are accounted for by its a) orbitals b) weight c) hydrogen bonds d) mass e) size
Answers: 2

Chemistry, 22.06.2019 14:30
The three types is stress that act on earths rocks are compression, tension, and
Answers: 1

Chemistry, 22.06.2019 23:00
Which of your 24 wells had indications that a chemical reaction occurred? how were you able to tell that a chemical reaction occurred? which of your 24 wells had indications that a physical reaction occurred? how were you able to tell that a physical reaction occurred? report on both mixing and evaporation. make a general statement about whether your hypotheses were validated or rejected. must your hypotheses be correct for this to be a successful laboratory?
Answers: 3

Chemistry, 23.06.2019 01:20
How can parts of a solution be separated by chromatography?
Answers: 1
You know the right answer?
On a balance, you have beakers of AgNO3AgNO3 solution and NaClNaCl solution. When mixed, they will f...
Questions













Computers and Technology, 16.11.2019 05:31







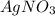 is added to NaCl then the compound formed will have same mass as that of reactants.
is added to NaCl then the compound formed will have same mass as that of reactants.