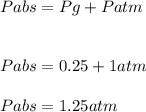Chemistry, 04.03.2020 03:27 akimadixon13
A ball has a volume of 5.27 liters and is at a temperature of 27.0°C. A pressure gauge attached to the ball reads 0.25 atmosphere. The atmospheric pressure is 1.00 atmosphere.
Calculate the absolute pressure inside the ball and the amount of air it contains.
The absolute pressure inside the ball is ??? atmospheres
The ball contains ???mole of air

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 12:30
A50.0 ml sample of gas at 20.0 atm of pressure is compressed to 40.0 atm of pressure at constant temperature. what is the new volume? 0.0100 ml 0.325 ml 25.0 ml 100. ml
Answers: 1

Chemistry, 23.06.2019 00:00
Mercury turns to a vapor at 629.88 k. how much heat is lost when 75.0 g of mercury vapor at 650 k condenses to a liquid at 297 k?
Answers: 1

Chemistry, 23.06.2019 00:30
The molecular weight of carbon dioxide, co2, is 44.00 amu, and the molecular weight of nitrous dioxide, no2, is 46.01 amu, so no2 diffuses co2
Answers: 2

You know the right answer?
A ball has a volume of 5.27 liters and is at a temperature of 27.0°C. A pressure gauge attached to t...
Questions




Mathematics, 22.05.2020 18:57

Computers and Technology, 22.05.2020 18:57



Mathematics, 22.05.2020 18:57








Mathematics, 22.05.2020 18:57

History, 22.05.2020 18:57


Mathematics, 22.05.2020 18:57