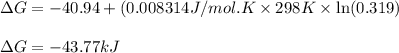Chemistry, 04.03.2020 01:51 gonzaleze18
Problem PageQuestion A chemist fills a reaction vessel with nitrogen monoxide gas, chlorine gas, and nitrosyl chloride gas at a temperature of . Under these conditions, calculate the reaction free energy for the following chemical reaction: Use the thermodynamic information in the ALEKS Data tab. Round your answer to the nearest kilojoule.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 23:50
2points why do scientists need governmental funding? o a. government politicians ask all the important scientific questions. o b. scientists have to pay taxes to the government on the money they make. o c. the cost of doing scientific research can be very high. o d. the government is controlled by scientists. submit
Answers: 3

Chemistry, 22.06.2019 05:00
Given sno2 + 2h2 - sn + 2h20 tin oxide reacts with hydrogen to produce tin and water. how many moles of sno2 are needed to produce 500.0 grams of sn?
Answers: 3


Chemistry, 22.06.2019 20:00
In vapor-liquid equilibrium in a binary mixture, both components are generally present in both phases. how many degrees of freedom are there for such a system? the reaction between nitrogen and hydrogen to form ammonia occurs in the gas phase. how many degrees of freedom are there for this system? steam and coal react at high temperatures to form hydrogen, carbon monoxide, carbon dioxide, and methane. the following reactions have been suggested as being involved in the chemical transformation:
Answers: 3
You know the right answer?
Problem PageQuestion A chemist fills a reaction vessel with nitrogen monoxide gas, chlorine gas, and...
Questions



Chemistry, 08.11.2019 06:31


Mathematics, 08.11.2019 06:31

English, 08.11.2019 06:31

English, 08.11.2019 06:31






Mathematics, 08.11.2019 06:31

Biology, 08.11.2019 06:31


Mathematics, 08.11.2019 06:31

English, 08.11.2019 06:31


English, 08.11.2019 06:31

Mathematics, 08.11.2019 06:31

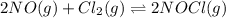
![\Delta G^o_{rxn}=\sum [n\times \Delta G^o_f_{(product)}]-\sum [n\times \Delta G^o_f_{(reactant)}]](/tpl/images/0533/0438/f2395.png)
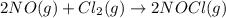
![\Delta G^o_{rxn}=[(2\times \Delta G^o_f_{(NOCl(g))})]-[(2\times \Delta G^o_f_{(NO(g))})+(2\times \Delta G^o_f_{(Cl_2)})]](/tpl/images/0533/0438/57ac5.png)
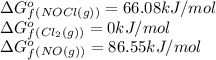
![\Delta G^o_{rxn}=[(2\times (66.08))]-[(2\times (86.55))+(1\times (0))]\\\\\Delta G^o_{rxn}=-40.94kJ](/tpl/images/0533/0438/c0c1f.png)
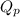 for above equation follows:
for above equation follows: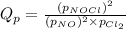
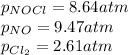
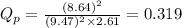
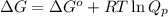
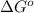 = standard Gibbs free energy change = -40.94 kJ
= standard Gibbs free energy change = -40.94 kJ![25^oC=[25+273]K=298K](/tpl/images/0533/0438/df1f6.png)