Consider the second-order reaction:
2HI(g)→H2(g)+I2(g)
Use the simulation to...

Chemistry, 03.03.2020 05:56 ayoismeisalex
Consider the second-order reaction:
2HI(g)→H2(g)+I2(g)
Use the simulation to find the initial concentration [HI]0 and the rate constant k for the reaction. What will be the concentration of HI after t = 4.53×1010 s ([HI]t) for a reaction starting under the condition in the simulation?
Given from simulation:
Rate Law: k[HI]^2
k= 6.4 x 10^-9 l/(mol x s) at 500K
Initial Rate= 1.6 x 10^-7 mol/(l x s)

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 23:00
The drawing represents the movement of particles in a substance. what changes of state can this substance undergo
Answers: 1

Chemistry, 22.06.2019 00:00
The p sub shell can hold up to 8 electrons in an atom. true or false?
Answers: 1

Chemistry, 22.06.2019 03:30
In saturated organic compounds, all the bonds between carbon atoms are called?
Answers: 1

Chemistry, 22.06.2019 07:00
In the cathode ray tube experiment, j. j. thomson passed an electric current through different gases inside a cathode ray tube in the presence of an electric field. in which two ways did this experiment change scientists’ understanding of the atom?
Answers: 2
You know the right answer?
Questions

Social Studies, 29.11.2020 16:50


Mathematics, 29.11.2020 16:50


Biology, 29.11.2020 16:50

Arts, 29.11.2020 16:50

Mathematics, 29.11.2020 16:50


Mathematics, 29.11.2020 16:50

Mathematics, 29.11.2020 16:50

Mathematics, 29.11.2020 16:50

Mathematics, 29.11.2020 16:50


Physics, 29.11.2020 16:50

Social Studies, 29.11.2020 16:50

Computers and Technology, 29.11.2020 16:50

English, 29.11.2020 16:50



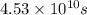 is 0.00345 mol/L.
is 0.00345 mol/L.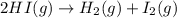
![k[HI]^2 ](/tpl/images/0531/9344/c44b5.png)
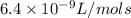
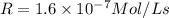
![[A_o]](/tpl/images/0531/9344/dc622.png)
![1.6\times 10^{-7} mol/L s=(6.4\times 10^{-9} L/mol s)[HI]^2](/tpl/images/0531/9344/8e7c2.png)
![[A_o]=5 mol/L](/tpl/images/0531/9344/ca62d.png)
![\frac{1}{[A]}=kt+\frac{1}{[A_o]}](/tpl/images/0531/9344/a4900.png)
![\frac{1}{[A]}=6.4\times 10^{-9} L/mol s\times 4.53\times 10^{10} s+\frac{1}{[5 mol/L]}](/tpl/images/0531/9344/3dcb7.png)
![[A]=0.00345 mol/L](/tpl/images/0531/9344/f652e.png)


