

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 14:00
The two naturally occurring isotopes of chlorine are 35cl (34.969 amu, 75.77%) and 37cl (36.966 amu, 24.23%). the two naturally occurring isotopes of bromine are 79br (78.918 rm amu, 50.69%) and 81br (80.916 amu, 49.31%). chlorine and bromine combine to form bromine monochloride, brcl. 1. how many peaks will be present in a mass spectrum for brcl? the four combinations of molecule possible given these four isotopes are: 81br37cl, 81br35cl, 79br37cl, and 79br35cl. 2. what are the masses of the four different brcl molecules? express the masses using six significant figures, in decreasing numeric order (highest to lowest), separated by commas.
Answers: 3

Chemistry, 22.06.2019 20:20
Which symbol can be used to indicate the pressure at which a chemical reaction is carried out? 25°c 2 atm pa
Answers: 2

Chemistry, 23.06.2019 00:30
What are the advantages of using the metric system? designed as a decimal system making conversions simpler more accurate system of measurement has prefixes that correspond to an amount to use with all base units used by the entire scientific community
Answers: 2

Chemistry, 23.06.2019 06:30
What type of chemical reaction occurs between silver nitrate (agno3) and copper (cu)? the equation i was given is 2agno3 + cu —> 2ag+ cu(no3)2.
Answers: 1
You know the right answer?
Assume that five weak acids, identified only by numbers (1, 2, 3, 4, and 5) have the following ioniz...
Questions

Mathematics, 22.05.2020 03:08










Mathematics, 22.05.2020 03:08

Mathematics, 22.05.2020 03:08



English, 22.05.2020 03:08





![K_a = \frac{[H^+]^2}{[HA]}](/tpl/images/0531/8279/683f8.png)
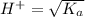 pH = -Log{H⁺]
pH = -Log{H⁺]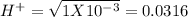 , pH = 1.5
, pH = 1.5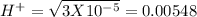 , pH = 2
, pH = 2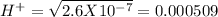 pH = 3
pH = 3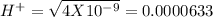 pH = 4
pH = 4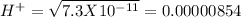 pH = 5
pH = 5 

