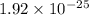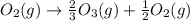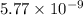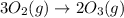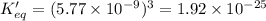Answers: 1
Another question on Chemistry


Chemistry, 22.06.2019 22:30
3.09 lab: reaction of metals 1 which combinations of substances resulted in a chemical change? for each metal that participated in a chemical change, write the type of metal it is, based on your examination of the periodic table. were there any metallic compounds that did not react with either the acid or the base? write the type of metal, based on your examination of the periodic table. make a general statement about the reactivity of the metals in this experiment.
Answers: 1

Chemistry, 23.06.2019 02:00
What can be done to make a solid solute dissolve faster in a liquid solvent?
Answers: 1

You know the right answer?
Given the value of the equilibrium constant (Kc) for the equation (a), calculate the equilibrium con...
Questions





Mathematics, 05.02.2021 02:50

Mathematics, 05.02.2021 02:50

Mathematics, 05.02.2021 02:50


Biology, 05.02.2021 02:50


Mathematics, 05.02.2021 02:50

History, 05.02.2021 02:50

Mathematics, 05.02.2021 02:50


Mathematics, 05.02.2021 02:50



Mathematics, 05.02.2021 02:50

Mathematics, 05.02.2021 02:50

Mathematics, 05.02.2021 02:50