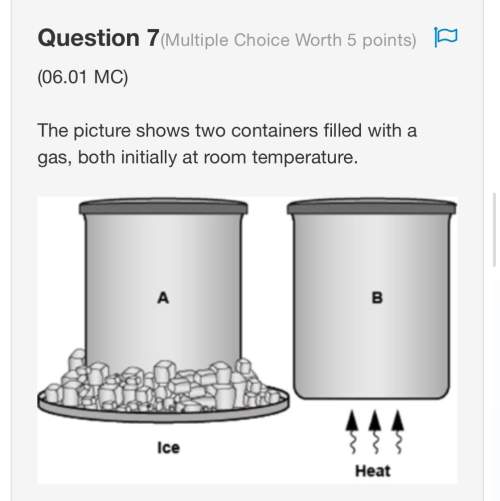
Chemistry, 03.03.2020 00:01 neverender098
The normal boiling point of ethanol is 78.4 °C and 101.3 KPa. The heat of vaporization for ethanol is 42.32 kJ/mol. Determine the vapor pressure of ethanol at 95.0 °C. Use the Clasius Clapeyron Equation: Ln [P2/P1] = - [∆Hvap /R] * [1/T2 - 1/T1]

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 06:00
Match the name of the following compound: mgso4 · h2omagnesium sulfate monohydratemagnesium (ii) sulfate monohydratemagnesium (ii) sulfate hydratemagnesium sulfate hydrate
Answers: 1

Chemistry, 22.06.2019 10:00
Part 1: include important facts found through your research. part 2: include your visual display. include your summary of “the chemistry of water” from the national science foundation website. include your experiment. part 3: include responses to the reflection questions.
Answers: 1

Chemistry, 22.06.2019 19:30
Acetylene gas c2h2 undergoes combustion to produce carbon dioxide and water vapor how many grams of water are produced by the same amount of c2h2?
Answers: 2

Chemistry, 22.06.2019 20:00
The volume of a single vanadium atom is 9.29×10-24 cm3. what is the volume of a vanadium atom in microliters?
Answers: 3
You know the right answer?
The normal boiling point of ethanol is 78.4 °C and 101.3 KPa. The heat of vaporization for ethanol i...
Questions



Mathematics, 24.02.2021 16:20



History, 24.02.2021 16:20

English, 24.02.2021 16:20


Geography, 24.02.2021 16:20


Mathematics, 24.02.2021 16:20



Mathematics, 24.02.2021 16:20

Mathematics, 24.02.2021 16:20



Social Studies, 24.02.2021 16:20

Mathematics, 24.02.2021 16:20

Mathematics, 24.02.2021 16:20