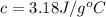Chemistry, 02.03.2020 19:22 PONBallfordM89
A 11.6 g piece of metal is heated to 98°C and dropped into a calorimeter containing 50.0 g of water (specific heat capacity of water is 4.18 J/g°C) initially at 20.5°C. The empty calorimeter has a heat capacity of 125 J/K. The final temperature of the water is 28.2°C. Ignoring significant figures, calculate the specific heat of the metal.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 09:00
Look at the spectrums of a star moving towards earth and a motionless star. which of these is a correct inference that can be draw from the observation of the two spectrums? (2 points) the spectrum of a motionless star is difficult to be viewed separately using oridinary telescopes. the spectrum of a motionless star is identical to the spectrum of a star which moves towards earth. the spectrum of a star shifts towards the red region when the star moves towards earth. the spectrum of a star shifts towards the blue region when the star moves towards earth.
Answers: 2

Chemistry, 22.06.2019 12:30
Place the elements below in order of decreasing ionization energy. aluminum(al) chlorine(cl) magnesium (mg) sulfur(s)
Answers: 1


Chemistry, 22.06.2019 22:30
Molecular iodine, i2(g), dissociates into iodine atoms at 625 k with a first-order rate constant of 0.271 s−1. part a part complete what is the half-life for this reaction?
Answers: 3
You know the right answer?
A 11.6 g piece of metal is heated to 98°C and dropped into a calorimeter containing 50.0 g of water...
Questions


Mathematics, 26.01.2022 15:00


Mathematics, 26.01.2022 15:00

Mathematics, 26.01.2022 15:00


Mathematics, 26.01.2022 15:00


Chemistry, 26.01.2022 15:00

Arts, 26.01.2022 15:10


Mathematics, 26.01.2022 15:10


Mathematics, 26.01.2022 15:10




Social Studies, 26.01.2022 15:10


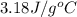
![q=-[q_1+q_2]](/tpl/images/0530/4537/f9283.png)
![m\times c\times (T_f-T_1)=-[c_1\times (T_f-T_2)+m_2\times c_2\times (T_f-T_2)]](/tpl/images/0530/4537/372b8.png)
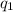 = heat absorbed by the calorimeter
= heat absorbed by the calorimeter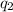 = heat absorbed by the water
= heat absorbed by the water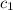 = specific heat of calorimeter =
= specific heat of calorimeter = 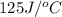
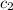 = specific heat of water =
= specific heat of water = 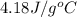
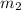 = mass of water = 50.0 g
= mass of water = 50.0 g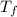 = final temperature =
= final temperature = 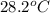
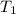 = temperature of metal =
= temperature of metal = 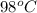
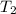 = temperature of water =
= temperature of water = 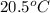
![11.6g\times c\times (28.2-98)^oC=-[125J/^oC\times (28.2-20.5)^oC+50.0g\times 4.18J/g^oC\times (28.2-20.5)^oC]](/tpl/images/0530/4537/17b20.png)