
2.1. Three moles of an ideal gas (with temperature-independent CP = (7/2)R, CV = (5/2)R) is contained in a horizontal piston/cylinder arrangement. The piston has an area of 0.1 m2 and mass of 500 g. The initial pressure in the piston is 101 kPa. Determine the heat that must be extracted to cool the gas from 375°C to 275°C at: (a) constant pressure; (b) constant volume.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 05:10
How many miles of water are produced if 5.43 mol pbo2 are consumed
Answers: 1

Chemistry, 22.06.2019 10:20
In a reaction equation, where are the products located? a.) above the arrow b.) to the right of the arrow c.) to the left of the arrow d.) below the arrow
Answers: 2

Chemistry, 22.06.2019 12:00
Hey guys so i need to know what is _nh3+> nh4oh ~chemistry~
Answers: 1

Chemistry, 22.06.2019 17:30
Upon decomposition, one sample of magnesium fluoride produced 1.65 kg of magnesium and 2.56 kg of fluorine. a second sample produced 1.32 kg of magnesium. part a how much fluorine (in grams) did the second sample produce?
Answers: 2
You know the right answer?
2.1. Three moles of an ideal gas (with temperature-independent CP = (7/2)R, CV = (5/2)R) is containe...
Questions





Health, 04.04.2020 04:00

English, 04.04.2020 04:00


Chemistry, 04.04.2020 04:00

Mathematics, 04.04.2020 04:00


History, 04.04.2020 04:00



Computers and Technology, 04.04.2020 04:00

Chemistry, 04.04.2020 04:00

Mathematics, 04.04.2020 04:00

Mathematics, 04.04.2020 04:00



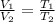 V₂ = T₂/T₁×V₁ = 548.15/648.15×0.11204 = 0.09476 m³
V₂ = T₂/T₁×V₁ = 548.15/648.15×0.11204 = 0.09476 m³

