
Chemistry, 28.02.2020 19:28 ballerboles4747
The radioisotope phosphorus-32 is used in tracers for measuring phosphorus uptake by plants. The half-life of phosphorus-32 is 14.3 days. If you begin with 30.5 mg of this isotope, what mass remains after 27.5 days have passed? Since the decomposition is a radioactive decay reaction, it is first order.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 01:30
100 points answer quick the table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 10 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of the two atoms? both are unreactive. both are highly reactive. a is unreactive and d is reactive. a is reactive and d is unreactive.
Answers: 1


Chemistry, 22.06.2019 09:30
One way that radioactive waste is treated is by burying it in repositories. the repositories are found only in states with very low populations. true or false? a. trueb. false(also i meant to put high school but it put down middle school instead)
Answers: 1

Chemistry, 22.06.2019 20:00
Which of the following would not diffuse through the plasma membrane by means of simple diffusion? 1 oxygen 2 glucose 3 a steroid hormone 4 a lipid soluble vitamin
Answers: 3
You know the right answer?
The radioisotope phosphorus-32 is used in tracers for measuring phosphorus uptake by plants. The hal...
Questions

English, 31.03.2021 21:10


Mathematics, 31.03.2021 21:10


Mathematics, 31.03.2021 21:10


Mathematics, 31.03.2021 21:10

Mathematics, 31.03.2021 21:10


Mathematics, 31.03.2021 21:10

Mathematics, 31.03.2021 21:10


Mathematics, 31.03.2021 21:10





Mathematics, 31.03.2021 21:10


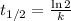
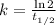
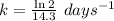
![[A_t]=[A_0]e^{-kt}](/tpl/images/0528/1963/1ef89.png)
![[A_t]](/tpl/images/0528/1963/5262c.png) is the concentration at time t
is the concentration at time t
![[A_0]](/tpl/images/0528/1963/9a686.png) is the initial concentration = 30.5 mg
is the initial concentration = 30.5 mg![[A_t]=30.5\times e^{-0.04847\times 27.5}\ mg=8.043\ mg](/tpl/images/0528/1963/500e7.png)


