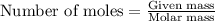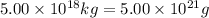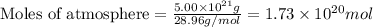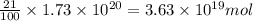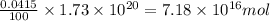The total mass of the atmosphere is about 5.00 x 1018 kg. How many moles each of air, O2, and CO2 are present in the atmosphere? Note that it is important to work in units of moles rather than in units of mass. By the ideal gas law, PV=nRT. P is pressure, V is volume, n is the number of moles, T is temperature (K), and R is the gas constant. At a given temperature and pressure, the volume is proportional to the number of moles, not to the mass.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 09:30
The chart shows the bid provided by four contractors to complete a job. which contractor is the most cost-effective?
Answers: 3

Chemistry, 22.06.2019 18:00
Many pharmaceutical drugs are organic compounds that were originally synthesized in the laboratory. which two scientific disciplines are bridged by pharmaceutical drugs? chemistry and forensics chemistry and medicine biology and forensics biology and criminology
Answers: 2

Chemistry, 23.06.2019 01:00
Which process results in the release of energy stored in the products of photosynthesis? a. polymer synthesis b. depolymerization c. digestion d. cellular respiration
Answers: 1

Chemistry, 23.06.2019 07:00
Determine the length of the object shown. 97.8 mm 97.80 mm 97 mm 98 mm
Answers: 1
You know the right answer?
The total mass of the atmosphere is about 5.00 x 1018 kg. How many moles each of air, O2, and CO2 ar...
Questions


Biology, 03.05.2021 21:40

History, 03.05.2021 21:40

English, 03.05.2021 21:40

Mathematics, 03.05.2021 21:40



Mathematics, 03.05.2021 21:40

Mathematics, 03.05.2021 21:40

English, 03.05.2021 21:40



Mathematics, 03.05.2021 21:40


Mathematics, 03.05.2021 21:40





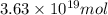 and
and 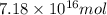 respectively
respectively