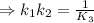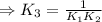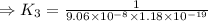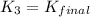Chemistry, 27.02.2020 00:10 barnhill6534
H2S(aq)⇌HS−(aq)+H+(aq), K1 = 9.06×10−8, and HS−(aq)⇌S2−(aq)+H+(aq), K2 = 1.18×10−19, what is the equilibrium constant Kfinal for the following reaction? S2−(aq)+2H+(aq)⇌H2S(aq)

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 04:30
How do i complete this electrolysis of water lab? i’m at home, so i don’t have the materials, and the lab didn’t properly work and was incomplete at school.
Answers: 1

Chemistry, 22.06.2019 06:30
The following reaction shows sodium carbonate reacting with calcium hydroxide. na2co3 + ca(oh)2 → naoh + caco3 how many grams of naoh are produced from 20.0 grams of na2co3? (molar mass of na = 22.989 g/mol, c = 12.01 g/mol, o = 15.999 g/mol, ca = 40.078 g/mol, h = 1.008 g/mol) 12.2 grams 15.1 grams 24.4 grams 30.2 grams
Answers: 2

Chemistry, 22.06.2019 14:00
How many absorptions would you expect to observe in the 13c nmr spectra of the following molecules? a) 3-chloropentane b) cis-4-methyl-2-pentene
Answers: 2

Chemistry, 22.06.2019 21:00
Use the measurements in the table to determine which unidentified metal has the highest density. metal volume mass a 10.5 cm3 122 g b 14.2 cm3 132 g c 16.1 cm3 115 g d 12.7 cm3 126 g
Answers: 2
You know the right answer?
H2S(aq)⇌HS−(aq)+H+(aq), K1 = 9.06×10−8, and HS−(aq)⇌S2−(aq)+H+(aq), K2 = 1.18×10−19, what is the equ...
Questions






Mathematics, 09.11.2020 16:20







Social Studies, 09.11.2020 16:20



Biology, 09.11.2020 16:20

Mathematics, 09.11.2020 16:20



Mathematics, 09.11.2020 16:20

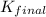 is 9.35× 10²⁵
is 9.35× 10²⁵![k=\frac{[C]^z}{[A]^x[B]^y}](/tpl/images/0525/8601/0a5aa.png)
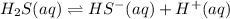
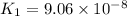
![K_1=\frac{[HS^-][H^+]}{[H_2S]}](/tpl/images/0525/8601/e1e1d.png)
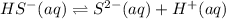
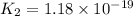
![K_2=\frac{[S^{2-}][H^+]}{[HS^-]}](/tpl/images/0525/8601/661de.png)
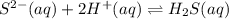
![K_3=\frac{[H_2S]}{[S^{2-}][H^+]^2}](/tpl/images/0525/8601/58537.png)
![k_1k_2=\frac{[HS^-][H^+]}{[H_2S]}\frac{[S^{2-}][H^+]}{[HS^-]}](/tpl/images/0525/8601/53894.png)
![\Rightarrow k_1k_2=\frac{[S^{2-}][H^+]^2}{[H_2S]}](/tpl/images/0525/8601/762f9.png)