
Chemistry, 26.02.2020 17:55 shadowselena63
The rate constant of a reaction is 6.85 × 10−5 L/mol·s at 195°C and 2.20 × 10−3 L/mol·s at 258°C. What is the activation energy of the reaction? Enter your answer in scientific notation.

Answers: 1
Another question on Chemistry


Chemistry, 22.06.2019 03:00
Explain how the integumentary system plays a crucial role in the ability to maintain homeoestasis
Answers: 1

Chemistry, 22.06.2019 16:50
Answer asap need by wednesday morning calculate the ph of 0.16m ch3cooh which has ka = 1.74 x 10-5 mol dm-3 best answer will be brainliest
Answers: 3

Chemistry, 22.06.2019 18:20
Which reason best explains why metals are malleable? a)because they have delocalized electrons b)because they have localized electrons c)because they have ionic bonds d)because they have rigid bonds
Answers: 2
You know the right answer?
The rate constant of a reaction is 6.85 × 10−5 L/mol·s at 195°C and 2.20 × 10−3 L/mol·s at 258°C. Wh...
Questions

English, 18.03.2021 03:20

Physics, 18.03.2021 03:20


History, 18.03.2021 03:20

Business, 18.03.2021 03:20

Mathematics, 18.03.2021 03:20

Mathematics, 18.03.2021 03:20

English, 18.03.2021 03:20

Mathematics, 18.03.2021 03:20

Mathematics, 18.03.2021 03:20

Mathematics, 18.03.2021 03:20

Mathematics, 18.03.2021 03:20


Mathematics, 18.03.2021 03:20

Mathematics, 18.03.2021 03:20

Chemistry, 18.03.2021 03:20

Mathematics, 18.03.2021 03:20


Mathematics, 18.03.2021 03:20

Mathematics, 18.03.2021 03:20

![\ln(\frac{K_{258^oC}}{K_{195^oC}})=\frac{E_a}{R}[\frac{1}{T_1}-\frac{1}{T_2}]](/tpl/images/0525/0415/d01a9.png)
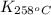 = equilibrium constant at 258°C =
= equilibrium constant at 258°C = 
 = equilibrium constant at 195°C =
= equilibrium constant at 195°C = 
 = Activation energy of the reaction = ?
= Activation energy of the reaction = ? = initial temperature =
= initial temperature = ![195^oC=[195+273]K=468K](/tpl/images/0525/0415/d4d73.png)
 = final temperature =
= final temperature = ![258^oC=[258+273]K=531K](/tpl/images/0525/0415/697bf.png)
![\ln(\frac{2.20\times 10^{-3}}{6.85\times 10^{-5}})=\frac{E_a}{8.314J/mol.K}[\frac{1}{468}-\frac{1}{531}]\\\\E_a=113780J/mol=113.8kJ/mol](/tpl/images/0525/0415/e4c71.png)


