
Chemistry, 26.02.2020 17:01 vanessasantos2004vs
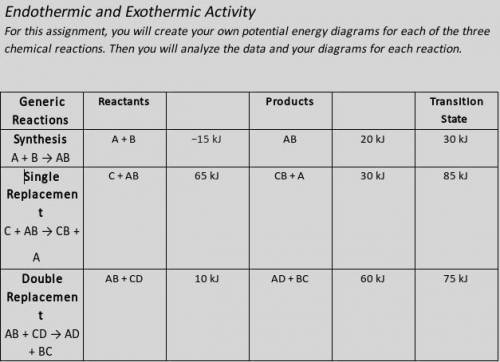
Illustrate the x- and y-axes to show the reaction pathway and potential energy, in kilojoules. Ensure your energy intervals are appropriate for the data.
Plot the enthalpy values of the reactants, products, and transition state using three horizontal dotted lines across the graph for each.
Draw the energy curve from the reactants line to the transition state and curve the line back down to the energy of the products. Label the reactants, products, and transition state.
Illustrate double-headed arrows to represent both the total change in enthalpy (ΔH) and the activation energy (Ea).
Calculate the total change in enthalpy and the activation energy using the energy values provided for each reaction. Record those values below the graph.
Make sure correct units are included.
I know this is a lot, so I'm giving more points than I ever have. Thank you!!!

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 05:50
What are transitions between a liquid and gas called? identify which way they are transitioning
Answers: 2

Chemistry, 22.06.2019 06:30
The minerals found in bones are deposited by living cells called
Answers: 1


Chemistry, 22.06.2019 14:40
Choose an equation that represents an enzyme-catalyzed reaction. (a) enzyme + substrate → enzyme-substrate complex (b) enzyme + substrate ←−→ enzyme + products (c) enzyme + substrate ←−→ enzyme-substrate complex → enzyme + products (d) enzyme + substrate ←−→ enzyme-substrate complex → enzyme-substrate complex + products
Answers: 2
You know the right answer?
Illustrate the x- and y-axes to show the reaction pathway and potential energy, in kilojoules. Ensur...
Questions

Mathematics, 29.09.2020 07:01


Mathematics, 29.09.2020 07:01

Mathematics, 29.09.2020 07:01

History, 29.09.2020 07:01


Mathematics, 29.09.2020 07:01








Mathematics, 29.09.2020 07:01

Mathematics, 29.09.2020 07:01


Mathematics, 29.09.2020 07:01


Mathematics, 29.09.2020 07:01



