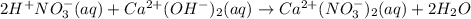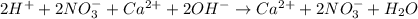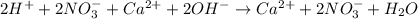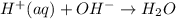Part A Write balanced molecular equation for the reaction between nitric acid and calcium hydroxide. Express your answer as a chemical equation. Identify all of the phases in your answer. nothing Request Answer Part B Enter a net ionic equation for the reaction between nitric acid and calcium hydroxide. Express your answer as a chemical equation. Identify all of the phases in your answer. nothing Request Answer

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 18:00
Construct the hypothetical phase diagram for metals a and b between room temperature (20c) and 700c, given the following information: * the melting temperature of metal a is 480c. • the maximum solubility of b in a is 4 wt% b, which occurs at 420c. • the solubility of b in a at room temperature is 0 wt% b. • one eutectic occurs at 420c and 18 wt% b–82 wt% a. • a second eutectic occurs at 475c and 42 wt% b–58 wt% a. • the intermetallic compound ab exists at a composition of 30 wt% b–70 wt% a, and melts congruently at 525c.• the melting temperature of metal b is 600c. • the maximum solubility of a in b is 13 wt% a, which occurs at 475c. • the solubility of a in b at room temperature is 3 wt% a.
Answers: 1

Chemistry, 21.06.2019 19:30
Agas has a volume of 0.7 l at 300 mmhg. what would be the new volume at 900 mmhg
Answers: 1

Chemistry, 22.06.2019 06:00
If you burn 10 kilograms of wood in a fire (combustion) what is the weight of the products after the fire has finished burning the wood?
Answers: 3

You know the right answer?
Part A Write balanced molecular equation for the reaction between nitric acid and calcium hydroxide....
Questions

Chemistry, 02.09.2021 22:10




Mathematics, 02.09.2021 22:10






Chemistry, 02.09.2021 22:10

Mathematics, 02.09.2021 22:10


Mathematics, 02.09.2021 22:10


English, 02.09.2021 22:10

English, 02.09.2021 22:10

Mathematics, 02.09.2021 22:10

Chemistry, 02.09.2021 22:10

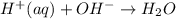 ". A further explanation is below.
". A further explanation is below.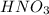 " and Calcium Hydroxide "
" and Calcium Hydroxide "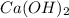 " gives Calcium Nitrate "
" gives Calcium Nitrate "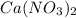 " and water "
" and water "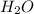 ".
".