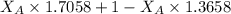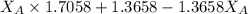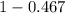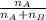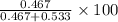A student was trying to determine the mole percent of A in a mixture of A and B using refractive index. If their mixture has a refractive index of 1.5248 and pure A and pure B each had refractive indices of 1.7058 and 1.3658, respectively, what was the mole percent of A in their mixture. Type your numerical answer rounded to the 3rd decimal place (i. e. 45.982 or 9.550, etc) without a percent sign.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 22:00
Your friend offers to show you an intrusive igneous rock. which of the following would you expect to see?
Answers: 1

Chemistry, 22.06.2019 13:00
What is the mass of 2.00 l of an intravenous glucose solution with a density of 1.15 g/ml?
Answers: 2

Chemistry, 22.06.2019 20:30
How many grams of phosphorus are contained in 5.09 moles of phosphorus?
Answers: 1

Chemistry, 22.06.2019 21:20
40dm3 of gas at 760 torr are heated from 5°c to 50°c what is the new volume
Answers: 3
You know the right answer?
A student was trying to determine the mole percent of A in a mixture of A and B using refractive ind...
Questions

Chemistry, 25.04.2020 03:15


Mathematics, 25.04.2020 03:15








Mathematics, 25.04.2020 03:15


English, 25.04.2020 03:15




Mathematics, 25.04.2020 03:16



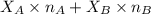
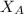 = mole fraction of A
= mole fraction of A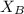 = mole fraction of B
= mole fraction of B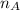 = refractive index of A
= refractive index of A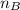 = refractive index of B
= refractive index of B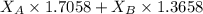 ........ (1)
........ (1)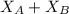 = 1
= 1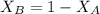 ......... (2)
......... (2)