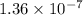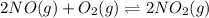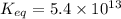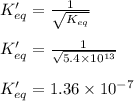Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 05:50
What happens when the temperature of a solution increases?
Answers: 2



Chemistry, 23.06.2019 15:30
In most resting cells, the concentration of sodium ions is higher outside of cells compared with the intracellular fluid. when cells are stimulated, sodium ion channels open, and sodium diffuses from the outside of the cell to the inside of the cell. sodium ion concentrations in a resting cell are an example of and sodium ion movement in a stimulated cell is an example of in most resting cells, the concentration of sodium ions is higher outside of cells compared with the intracellular fluid. when cells are stimulated, sodium ion channels open, and sodium diffuses from the outside of the cell to the inside of the cell. sodium ion concentrations in a resting cell are an example of and sodium ion movement in a stimulated cell is an example of potential energy; kinetic energy kinetic energy; potential energy the energy of motion; stored energy chemical work; energy stored in chemical bonds
Answers: 2
You know the right answer?
The Keq for the equilibrium below is 5.4 × 1013 at 480.0 °C. 2NO (g) + O2 (g) 2NO2 (g) What is the v...
Questions

Social Studies, 08.12.2020 20:00

Mathematics, 08.12.2020 20:00


English, 08.12.2020 20:00

Mathematics, 08.12.2020 20:00

Mathematics, 08.12.2020 20:00

Mathematics, 08.12.2020 20:00


Chemistry, 08.12.2020 20:00

Mathematics, 08.12.2020 20:00


Mathematics, 08.12.2020 20:00



History, 08.12.2020 20:00

Computers and Technology, 08.12.2020 20:00



Mathematics, 08.12.2020 20:00

Biology, 08.12.2020 20:00

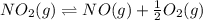 equation is
equation is