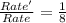Chemistry, 25.02.2020 04:05 alialbinn6969
The gas phase reaction between NO and O2 is second order with respect to NO and first order with respect to 02, what would happen to the reaction rate if the concentrations of both reactants were halved with everything else held constant?
a. It would decrease by a factor of 8 It would remain unchanged.
b. It would decrease by a factor of 16.
c. It would decrease by a factor of 4.
d. It would decrease by a factor of 2

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 21:30
Balance this equation co2(g) + h2o (g) show that the balanced equation obeys the law if conversation of mass
Answers: 1

Chemistry, 22.06.2019 08:40
For each of the following compounds, write the formula then predict whether it would be a strong, weak, or non-electrolyte when placed in di water. for the ionic compounds only, put (s) or (aq) after the forrmula formula strong, weak or non electrolyte? a calcium hydroxide b. silver carbonate c. lead(ii) sulfate d. phosphorus trifluoride e. sodium phosphide f barium sulfate g. strontium acetate h. zinc nitrate
Answers: 3

Chemistry, 22.06.2019 15:00
20 pts ‼️ an unmanned spacecraft travels to mars. mars has a lower strength of gravity than earth. where in the image is the spacecraft’s weight the greatest?
Answers: 1

Chemistry, 23.06.2019 04:00
What is the volume of 2.5 moles of nitrogen gas (n2)at standard temperature and pressure (stp)?
Answers: 1
You know the right answer?
The gas phase reaction between NO and O2 is second order with respect to NO and first order with res...
Questions








Mathematics, 29.09.2020 14:01

Mathematics, 29.09.2020 14:01



Mathematics, 29.09.2020 14:01

Computers and Technology, 29.09.2020 14:01

English, 29.09.2020 14:01

Mathematics, 29.09.2020 14:01

Mathematics, 29.09.2020 14:01


History, 29.09.2020 14:01


Mathematics, 29.09.2020 14:01

![Rate=k[NO]^2[O_2]^1](/tpl/images/0522/7517/1df0d.png) (1)
(1)![Rate'=k{\frac{[NO]}{2}^2}{\frac{[O_2]}{2}^1}](/tpl/images/0522/7517/19c10.png) (2)
(2)![\frac{Rate'}{Rate}=\frac{k\frac{[NO]}{2}^2\times \frac{([O_2]}{2})^1}{k[NO]^2[O_2]^1}](/tpl/images/0522/7517/9c613.png)