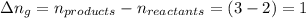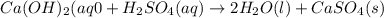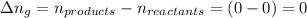Chemistry, 25.02.2020 03:04 DaFuzzyDude
The enthalpy change, ΔH, for a reaction at constant pressure is defined as: ΔH = ΔE + PΔV. For which of the following reactions will ΔH be approximately equal to ΔE? Select all that apply. Group of answer choices 2 NO2(g) -> N2(g) + 2 O2(g)

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 17:10
There are 6.022 x 10^23 atoms of hg in 1 mole of hg. the number of atoms in 4.5 moles of hg can be found by multiplying 4.5 by 6.022 x 10^23 a. 2.7 x 10^24 b. 27 x 10^23 c. 2.71 x10^24 d. 27.099 x 10^23
Answers: 3

Chemistry, 22.06.2019 07:10
Provide a stepwise curved arrow mechanism that fully explains the outcome of the reaction shown below. oh нао* heat он
Answers: 2

Chemistry, 22.06.2019 15:30
The gulf stream is a warm water current that flows away from the equator to northern europe. witch of these does it cause. a. crashes of warm and cool water in the ocean b.colder climates near the equator c.large waves on the cost of europe d.warm climates in northern europe
Answers: 1

Chemistry, 22.06.2019 23:00
In which region is the substance in both the solid phase and the liquid phase? 1 2. 3 4 mark this and return save and exit next
Answers: 2
You know the right answer?
The enthalpy change, ΔH, for a reaction at constant pressure is defined as: ΔH = ΔE + PΔV. For which...
Questions


Business, 29.07.2019 19:30


Mathematics, 29.07.2019 19:30

History, 29.07.2019 19:30

History, 29.07.2019 19:30


Mathematics, 29.07.2019 19:30


History, 29.07.2019 19:30


Mathematics, 29.07.2019 19:30


Computers and Technology, 29.07.2019 19:30

Mathematics, 29.07.2019 19:30

English, 29.07.2019 19:30


Mathematics, 29.07.2019 19:40


Chemistry, 29.07.2019 19:40

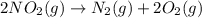
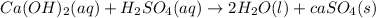
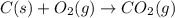
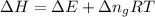 as
as 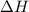 = enthalpy change
= enthalpy change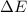 = internal energy change
= internal energy change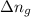 = change in number of moles of gas particles =
= change in number of moles of gas particles =