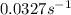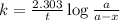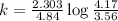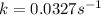Chemistry, 24.02.2020 23:41 davisbrittany5784
Consider the following first-order reaction: A → B. The concentration of A at the start of the reaction is 4.17 M and after 4.84 s is 3.56 M. (a) Using the integrated rate law for a first-order reaction, calculate the value of the rate constant.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 07:00
The blackbody curve for a star name zeta is shown below. what is the peak wavelength for this star ?
Answers: 1

Chemistry, 22.06.2019 08:30
Analyze how limestone is weathered and identify the features that are formed as a result of this dissolution
Answers: 1

Chemistry, 22.06.2019 15:00
20 pts ‼️ an unmanned spacecraft travels to mars. mars has a lower strength of gravity than earth. where in the image is the spacecraft’s weight the greatest?
Answers: 1

Chemistry, 22.06.2019 22:00
What mass of glucose is produced when 54g of water react with carbon dioxide
Answers: 1
You know the right answer?
Consider the following first-order reaction: A → B. The concentration of A at the start of the react...
Questions

History, 09.09.2021 06:40






Mathematics, 09.09.2021 06:40

English, 09.09.2021 06:40

Mathematics, 09.09.2021 06:40

Mathematics, 09.09.2021 06:40




Mathematics, 09.09.2021 06:40

World Languages, 09.09.2021 06:40

English, 09.09.2021 06:40

Mathematics, 09.09.2021 06:40