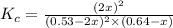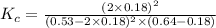Consider the reaction between NONO and Cl2Cl2 to form NOClNOCl: 2NO(g)+Cl2(g)⇌2NOCl(g)2NO(g)+Cl2(g) ⇌2NOCl(g) A reaction mixture at a certain temperature initially contains only [NO]=[NO]= 0.53 MM and [Cl2]=[Cl2]= 0.64 MM. After the reaction comes to equilibrium, the concentration of NOClNOCl is 0.36 MM. Part A Find the value of the equilibrium constant (Kc)(Kc) at this temperature.

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 15:30
How many atoms are in 1.4 mil of phosphorus trifluoride (pf3)
Answers: 3

Chemistry, 21.06.2019 16:00
Nickel crystallizes in the face-centered cubic (fcc) lattice. the density of the metal is 8902 kg/m3. calculate the radius of a nickel atom.
Answers: 1

Chemistry, 21.06.2019 19:30
The molecular formula for caffeine is cshion402. which of the following elements is not found in caffeine?
Answers: 1

Chemistry, 22.06.2019 04:30
Which statement best describes the relationship between period and frequency of light waves? a) in wave b the period increases and the frequency decreases from wave a. b) in wave a the period increases and the frequency decreases from wave b. c) in wave b the period is shorter and the frequency is greater than in wave a. d) in wave a the period is shorter and the frequency is greater than in wave b.
Answers: 1
You know the right answer?
Consider the reaction between NONO and Cl2Cl2 to form NOClNOCl: 2NO(g)+Cl2(g)⇌2NOCl(g)2NO(g)+Cl2(g)...
Questions


Mathematics, 18.11.2019 09:31

Mathematics, 18.11.2019 09:31

Mathematics, 18.11.2019 09:31

Mathematics, 18.11.2019 09:31

Mathematics, 18.11.2019 09:31



Mathematics, 18.11.2019 09:31

Mathematics, 18.11.2019 09:31

English, 18.11.2019 09:31

Mathematics, 18.11.2019 09:31

Mathematics, 18.11.2019 09:31

Mathematics, 18.11.2019 09:31

Mathematics, 18.11.2019 09:31





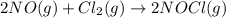
![K_c=\frac{[NOCl]^2}{[NO]^2[Cl_2]}](/tpl/images/0521/6978/56950.png)