
Chemistry, 24.02.2020 18:53 jaylennkatrina929
A certain second-order reaction (B→products) has a rate constant of 1.30×10−3 M−1⋅s−1 at 27 ∘C and an initial half-life of 224 s . What is the concentration of the reactant B after one half-life?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 00:10
According to the diagram; a) identify the anode of the cell and write the half-reaction that occurs there b) write the overall equation for the reaction that occurs as the cell operates c) calculate the value of the standard cell potential ,e cell. d)write the shorthand notation of the cell above e)indicate the flow of the electrons on the diagram
Answers: 3

Chemistry, 22.06.2019 01:00
Which part of a feedback mechanism is able to monitor the conditions outside of cells and usually uses nerve cells to relay this information to an intergrating center
Answers: 2


Chemistry, 22.06.2019 21:00
One similarity and one difference between an element and a mixture of elements
Answers: 1
You know the right answer?
A certain second-order reaction (B→products) has a rate constant of 1.30×10−3 M−1⋅s−1 at 27 ∘C and a...
Questions



Computers and Technology, 03.09.2021 01:10


English, 03.09.2021 01:10


Mathematics, 03.09.2021 01:10




Mathematics, 03.09.2021 01:20

Social Studies, 03.09.2021 01:20





Mathematics, 03.09.2021 01:20



Mathematics, 03.09.2021 01:20


![\dfrac{d[B]}{dt}=-k[B]^2](/tpl/images/0521/4312/89d63.png)
![\dfrac{1}{[B]}=\dfrac{1}{[B]_0}+kt](/tpl/images/0521/4312/c6bcf.png)
![t_{1/2}=\dfrac{1}{k[A]_0}](/tpl/images/0521/4312/90d03.png)
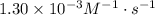 and the half-life time 224s to find [A]₀:
and the half-life time 224s to find [A]₀:![224s=\dfrac{1}{1.30\times10^{-3}M^{-1}\cdot s^{-1}[A]_0}](/tpl/images/0521/4312/bcef9.png)
![[A]_o=0.291M](/tpl/images/0521/4312/294dc.png)


