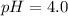Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 06:00
Ethanol (c2h5oh) is produced from the fermentation of sucrose in the presence of enzymes. c12h22o11(aq) + h2o(g) 4 c2h5oh(l) + 4 co2(g) determine the theoretical yield and the percent yields of ethanol if 680. g sucrose undergoes fermentation and 326.5 g ethanol is obtained. theoretical _ g _ percent %
Answers: 1

Chemistry, 22.06.2019 07:30
In a reaction (at equilibrium) that makes more moles of gas than it consumes, what is the effect of increasing the pressure?
Answers: 1

Chemistry, 22.06.2019 09:20
Which of these statements explains the difference between nuclear binding energy and the strong nuclear force ?
Answers: 3

Chemistry, 22.06.2019 19:00
Suppose that a certain fortunate person has a net worth of $71.0 billion ($7.10×1010). if her stock has a good year and gains $3.20 billion (3.20×109) in value, what is her new net worth?
Answers: 3
You know the right answer?
Calculate the pH of a solution that is 0.240 M in sodium formate (HCOONa) and 0.120 M in formic acid...
Questions

Chemistry, 12.01.2020 04:31

History, 12.01.2020 04:31

Mathematics, 12.01.2020 04:31

Chemistry, 12.01.2020 04:31

Mathematics, 12.01.2020 04:31

Mathematics, 12.01.2020 04:31

Health, 12.01.2020 04:31


Physics, 12.01.2020 04:31



Chemistry, 12.01.2020 04:31



Mathematics, 12.01.2020 04:31



Mathematics, 12.01.2020 05:31


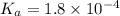
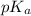 .
.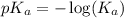
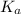 in this expression, we get:
in this expression, we get: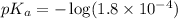
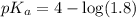
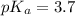
![pH=pK_a+\log \frac{[Salt]}{[Acid]}](/tpl/images/0521/4495/e961a.png)