

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 07:00
This image is an example of a(n) a) atom. b) compound. c) mixture. d) molecule.
Answers: 1

Chemistry, 22.06.2019 19:00
Nan element’s square on the periodic table, the number with the greatest numerical value represents the
Answers: 3

Chemistry, 22.06.2019 22:00
Scientists often have to deal with numbers that are either very large or very small. for example, the radius of the sun is approximately 696,000 kilometers, while bacterial cells are as small as 1.9 × 10-4 millimeters. express each number in an alternate form.
Answers: 1

Chemistry, 22.06.2019 22:30
Which one of the following bonds would you expect to be the most polar? a) b–h b) n–h c) p–h d) al–h e) c–h
Answers: 1
You know the right answer?
Consider the following equilibrium for nitrous acid, HNO2, a weak acid: HNO2(aq) + H2O (l) —><...
Questions



Mathematics, 22.04.2020 01:34

Biology, 22.04.2020 01:34


History, 22.04.2020 01:34

Mathematics, 22.04.2020 01:34

Mathematics, 22.04.2020 01:34



Biology, 22.04.2020 01:34









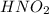 is weaker acid and have less tendency to dissociate in water.Hence, formation of reactants will be more favourable than the formation of products.When the acid is more diluted more number of hydronium ions are produced hence the direction of equilibrium will shift to forward direction.
is weaker acid and have less tendency to dissociate in water.Hence, formation of reactants will be more favourable than the formation of products.When the acid is more diluted more number of hydronium ions are produced hence the direction of equilibrium will shift to forward direction.

