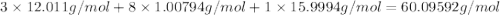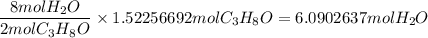Chemistry, 24.02.2020 07:43 bridgettebach
When 91.5 g of isopropyl alcohol which has an empirical formula of C3H8O is burned in excess oxygen gas, how many grams of H2O are formed? MWC = 12.011 g/mol, MWH = 1.00794 g/mol, and MWO = 15.9994 g/mol.
1. 47.9229
2. 84.1255
3. 52.2617
4. 49.8948
5. 86.9152
6. 119.705
7. 88.0758
8. 76.2076
9. 62.9663
10. 109.729

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 01:40
Which characteristic of water it form droplets? a. low specific heat b. nonpolar structure c. high surface tension d. ability to dissolve substances
Answers: 1

Chemistry, 22.06.2019 10:30
Great amounts of electromagnetic energy from our sun and other bodies in space travel through space. which is a logical conclusion about these electromagnetic waves? their energy must be very their frequency must be very low these waves can travel without a medium they only travel through a vacuum of space
Answers: 2


Chemistry, 23.06.2019 13:30
Malik formed a hypothesis that an increase in atmospheric oxygen levels by 10% would cause red-legged grasshoppers to grow larger than normal. suppose that malik performs an experiment to test his hypothesis. which of these actions would represent a scientific mistake in his experiment? a. he experiments on live grasshoppers instead of preserved ones. b. he focuses on red-legged grasshoppers instead of all kinds of grasshoppers. c. he varies the nitrogen and carbon dioxide levels in the air from one trial to the next. d. he conducts the experiment in a controlled lab setting with a lab partner. e. he measures the mass and length of his specimens at the start of each trial.
Answers: 1
You know the right answer?
When 91.5 g of isopropyl alcohol which has an empirical formula of C3H8O is burned in excess oxygen...
Questions


English, 06.05.2020 20:15

Mathematics, 06.05.2020 20:15

Mathematics, 06.05.2020 20:15



Mathematics, 06.05.2020 20:16






English, 06.05.2020 20:16







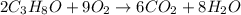
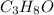 produce 8 moles of
produce 8 moles of 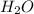 .
.