Chemistry, 23.02.2020 00:22 JamierW2005
Using the symbol below/above determine the number of protons, neutrons, and electrons in the element.
A. Protons = 50
Neutrons = 118
Protons = 46
B. Protons = 68
Neutrons = 46
Electrons = 54
C. Protons = 50
Neutrons = 68
Electrons = 46
D. Protons = 118
Neutrons = 50
Electrons = 46
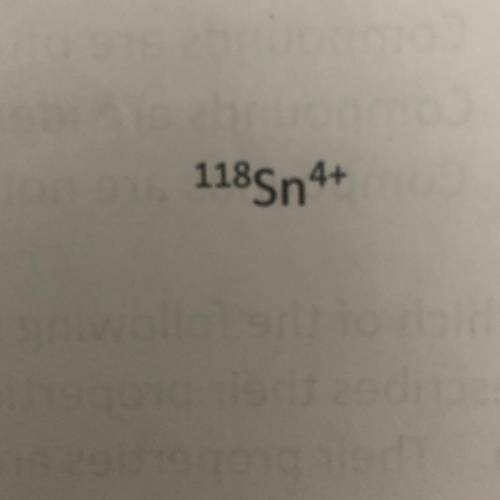

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 06:00
There are 6.022, 104 atoms of hg in 1 mole of hg the number of atoms in 45 moles of hg can be found by multiplying 4.5 by 6.022, 102 which is the number of atoms in 4.5 moles of hg, correctly written in scientific notation with the correct number of significant figures? 0 21,109 0 21,100 271, 1024 27.099, 100 mark this and retum save and exit submit
Answers: 1

Chemistry, 22.06.2019 07:30
Using data from seismic waves, geologists have learned that earth’s interior is made up of several
Answers: 3

Chemistry, 22.06.2019 13:20
Can someone me with 3 and 4 plz. this is for masteries test.
Answers: 2

Chemistry, 22.06.2019 13:30
Which statements are true concerning mineral formation? check all that apply. the slower the cooling, the larger the crystals. the faster the cooling, the smaller the crystals. crystals formed from magma are smaller than crystals formed from lava. minerals can only form in solutions when the solution is heated deep underground. when a solution cools, elements and compounds leave the solution and crystallize as minerals. minerals formed from hot water solutions can form narrow channels in the surrounding rock.
Answers: 1
You know the right answer?
Using the symbol below/above determine the number of protons, neutrons, and electrons in the element...
Questions



Computers and Technology, 06.08.2021 17:20



Mathematics, 06.08.2021 17:20

Advanced Placement (AP), 06.08.2021 17:20


English, 06.08.2021 17:20


Physics, 06.08.2021 17:20

Mathematics, 06.08.2021 17:20

Physics, 06.08.2021 17:20