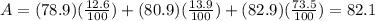Chemistry, 22.02.2020 04:36 sarahgarza5440
What is the relative atomic mass of a hypothetical element that consists of the following isotopes in the indicated natural abundances? Isotope - Isotopic mass (amuamu) - Relative abundance (%%) 1 - 78.9 - 12.6 2 - 80.9 - 13.9 3 - 82.9 - 73.5

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 09:00
Scientific evidence tells us that the cause of earths four season is the tilt of earth as it revolves around the sun. the student is instructed to illustrate this information in a science notebook. how will the student illiterate winter in the northern hemisphere?
Answers: 3


Chemistry, 22.06.2019 10:30
Which of these is not an example of chemical weathering? a. iron-rich mineral rusting b. feldspar turning into clay c. limestone reacting with acid d. granite breaking up into sand
Answers: 1

Chemistry, 22.06.2019 15:00
Which theory was contradicted by experiments with the photoelectric effect? light spreads out after it passes through a small opening. as soon as light strikes metal, electrons will be ejected. visible light, regardless of color, will cause the ejection of electrons when striking metal. the kinetic energy of ejected electrons depends on the frequency of light that strikes the metal.
Answers: 2
You know the right answer?
What is the relative atomic mass of a hypothetical element that consists of the following isotopes i...
Questions

Mathematics, 13.01.2021 09:40

Mathematics, 13.01.2021 09:40

English, 13.01.2021 09:40


Mathematics, 13.01.2021 09:40

History, 13.01.2021 09:40

English, 13.01.2021 09:40

Mathematics, 13.01.2021 09:40


Social Studies, 13.01.2021 09:50


Geography, 13.01.2021 09:50



English, 13.01.2021 09:50


Mathematics, 13.01.2021 09:50

English, 13.01.2021 09:50


Chemistry, 13.01.2021 09:50

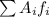
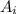 is the atomic mass of each isotope and
is the atomic mass of each isotope and  the relative frequency. Therefore, we find:
the relative frequency. Therefore, we find: