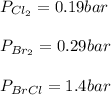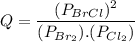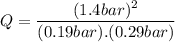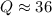Chemistry, 21.02.2020 23:49 Katlynnmarkle13
Consider the gas-phase reaction, Cl2(g) + Br2(g) <=> 2 BrCl(g), for which Kp = 32 at 500 K. If the mixture is analyzed and found to contain 0.19 bar of Cl2, 0.29 bar of Br2 and 1.4 bar of BrCl, describe the situation:a) Q > K and more reactants will be made to reach equilibrium. b) Q > K and more products will be made to reach equilibrium. c) Within 1 decimal place, Q = K and the reaction is at equilibriumd) Q < K and more products will be made to reach equilibrium. e) Q < K and more reactants will be made to reach equilibrium.

Answers: 2
Another question on Chemistry


Chemistry, 22.06.2019 03:30
Explain why pure hydrogen cyanide does not conduct electricity, but become a conductor when it is dissolved in water? (at room temp, pure hcn exists as a volatile liquid)
Answers: 1

Chemistry, 22.06.2019 10:00
Main expenses you plan on making payments on a new car too. you want to spend 15% of your monthly net pay on the car payment, insurance, registration, and taxes combined. what is your monthly car allowance? $149.46 $298.91 $448.37 $597.83
Answers: 3

Chemistry, 23.06.2019 01:30
What is produced from neutralization of an acid and a base? a. hydronium ions b. citric acid c. salt and water
Answers: 1
You know the right answer?
Consider the gas-phase reaction, Cl2(g) + Br2(g) <=> 2 BrCl(g), for which Kp = 32 at 500 K. If...
Questions


History, 27.01.2021 20:00




Biology, 27.01.2021 20:00




History, 27.01.2021 20:00


English, 27.01.2021 20:00






Mathematics, 27.01.2021 20:00