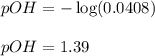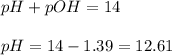An analytical chemist is titrating 242.5mL of a 1.200M solution of hydrazoic acid HN3 with a 0.3400M solution of NaOH . The pKa of hydrazoic acid is 4.72 . Calculate the pH of the acid solution after the chemist has added 1006.mL of the NaOH solution to it. Note for advanced students: you may assume the final volume equals the initial volume of the solution plus the volume of NaOH solution added. Round your answer to 2 decimal places.

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 14:10
The rock in a lead ore deposit contains 89 % pbs by mass. how many kilograms of the rock must be processed to obtain 1.5 kg of pb?
Answers: 1

Chemistry, 21.06.2019 14:20
Which of the following statements is not true? • a. covalent compounds have low melting and boiling points. • ob. covalent bonds between atoms of a compound are relatively weak compared to bonds between molecules. • c. covalent bonds occur between nonmetals. • d. covalent compounds are often gases or liquids.
Answers: 2

Chemistry, 21.06.2019 22:30
Hot air balloons float in the air because of the difference in density between cold and hot air. in this problem, you will estimate the minimum temperature the gas inside the balloon needs to be, for it to take off. to do this, use the following variables and make these assumptions: the combined weight of the pilot basket together with that of the balloon fabric and other equipment is w. the volume of the hot air inside the balloon when it is inflated is v. the absolute temperature of the hot air at the bottom of the balloon is th (where th> tc). the absolute temperature of the cold air outside the balloon is tc and its density is ďc. the balloon is open at the bottom, so that the pressure inside and outside the balloon is the same. as always, treat air as an ideal gas. use g for the magnitude of the acceleration due to gravity.
Answers: 1

Chemistry, 22.06.2019 14:00
8.98 dm3 of hydrogen gas is collected at 38.8 °c. find the volume the gas will occupy at -39.9 °c if the pressure remains constant.
Answers: 3
You know the right answer?
An analytical chemist is titrating 242.5mL of a 1.200M solution of hydrazoic acid HN3 with a 0.3400M...
Questions





Mathematics, 28.01.2020 02:31












Mathematics, 28.01.2020 02:31



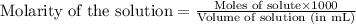 ......(1)
......(1)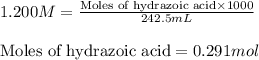
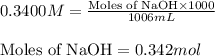
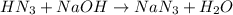
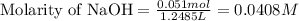
![pOH=-\log[OH^-]](/tpl/images/0519/7873/fe336.png)
![[OH^-]=0.0408M](/tpl/images/0519/7873/8744d.png)