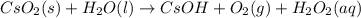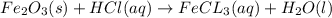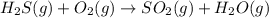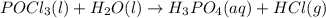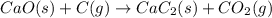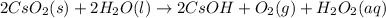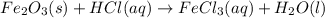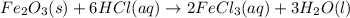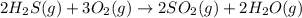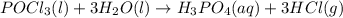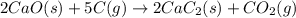Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 00:00
Which of the following methods uses the decay of atomic particles in an object to find its exact age? a. fossil dating b. geologic dating c. radioactive dating d. relative dating
Answers: 1

Chemistry, 22.06.2019 05:30
According to periodic trend, which of the following most likely has the highest ionization energy? kr be ni sc
Answers: 3

Chemistry, 22.06.2019 10:50
8) a mixture of he, ne and ar has a pressure of 7.85 atm. if the ne has a mole fraction of 0.47 and 8) ar has a mole fraction of 0.23, what is the pressure of he? a) 4.2 atm b) 3.7 atm c) 5.5 atm d) 2.4 atm e) 1.8 atm
Answers: 1

Chemistry, 22.06.2019 13:00
The number of neutrons is equal to the atomic number minus the atomic mass. a. true b. false
Answers: 2
You know the right answer?
Balance each of the following chemical equations. (Use the lowest possible coefficients for all reac...
Questions


Mathematics, 10.05.2021 09:20




Mathematics, 10.05.2021 09:20


Mathematics, 10.05.2021 09:20




Mathematics, 10.05.2021 09:20

History, 10.05.2021 09:20


Social Studies, 10.05.2021 09:20

Mathematics, 10.05.2021 09:20

Physics, 10.05.2021 09:20


Business, 10.05.2021 09:20

Mathematics, 10.05.2021 09:20