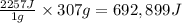Chemistry, 21.02.2020 02:28 akatian55721
As an athlete exercises, sweat is produced and evaporated to help maintain a proper body temperature. On average, an athlete loses approximately 307 g of sweat during an hour of exercise. How much energy is needed to evaporate the sweat that is produced? The heat of vaporization for water is 2257J/g. energy required:

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 20:40
Which of the following pressures is equal to 760 mm hg? 2.0 atm 101.3 pa 101,300 kpa 101,300 pa
Answers: 2


Chemistry, 22.06.2019 04:30
Which statement best describes the relationship between period and frequency of light waves? a) in wave b the period increases and the frequency decreases from wave a. b) in wave a the period increases and the frequency decreases from wave b. c) in wave b the period is shorter and the frequency is greater than in wave a. d) in wave a the period is shorter and the frequency is greater than in wave b.
Answers: 1

Chemistry, 22.06.2019 05:30
What type of reaction is shown below? check all that apply. 2h2o2 → 2h2o + o2 synthesis decomposition combustion
Answers: 1
You know the right answer?
As an athlete exercises, sweat is produced and evaporated to help maintain a proper body temperature...
Questions

Social Studies, 27.09.2019 06:30

History, 27.09.2019 06:30

Biology, 27.09.2019 06:30

Biology, 27.09.2019 06:30

English, 27.09.2019 06:30


Mathematics, 27.09.2019 06:30

History, 27.09.2019 06:30




Mathematics, 27.09.2019 06:30






Geography, 27.09.2019 06:30

Mathematics, 27.09.2019 06:30

Chemistry, 27.09.2019 06:30