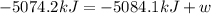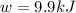Chemistry, 21.02.2020 02:32 smusisca53
The change in internal energy for the combustion of 1.0 mol of octane at a pressure of 1.0 atm is -5084.1 kJ. You may want to reference (Pages 381 - 385) Section 9.6 while completing this problem.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 08:30
How would the number of moles (n) of o2 change if the atmospheric pressure doubled but all other variables stayed the same
Answers: 2

Chemistry, 22.06.2019 20:00
Which of the following would not diffuse through the plasma membrane by means of simple diffusion? 1 oxygen 2 glucose 3 a steroid hormone 4 a lipid soluble vitamin
Answers: 3

Chemistry, 22.06.2019 20:30
Which states of matter have particles that move independently of one another with very little attraction?
Answers: 1

Chemistry, 22.06.2019 21:20
If a simple machine aduces the strength of a force, what must be increased? the speed of the input force the work the simple machine performs the size of the simple machine the distance over which the force is applied
Answers: 1
You know the right answer?
The change in internal energy for the combustion of 1.0 mol of octane at a pressure of 1.0 atm is -5...
Questions

Biology, 24.11.2019 18:31



Biology, 24.11.2019 19:31







Mathematics, 24.11.2019 19:31

English, 24.11.2019 19:31





Mathematics, 24.11.2019 19:31


Mathematics, 24.11.2019 19:31


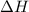 = change in enthalpy = -5074.2 kJ
= change in enthalpy = -5074.2 kJ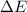 = change in internal energy = -5084.1 kJ
= change in internal energy = -5084.1 kJ